https://www.cup.lmu.de/pb/aks/merkel/
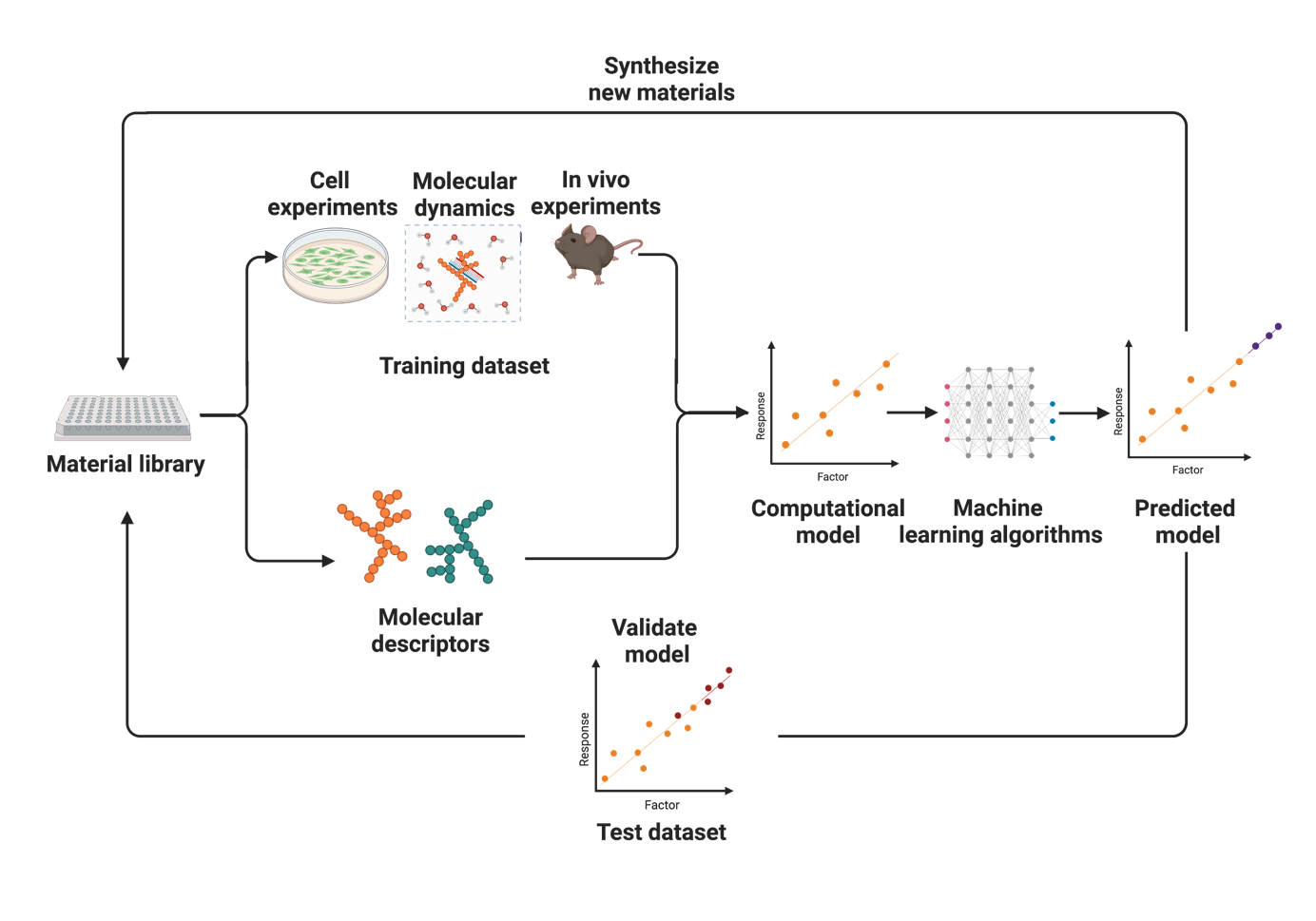
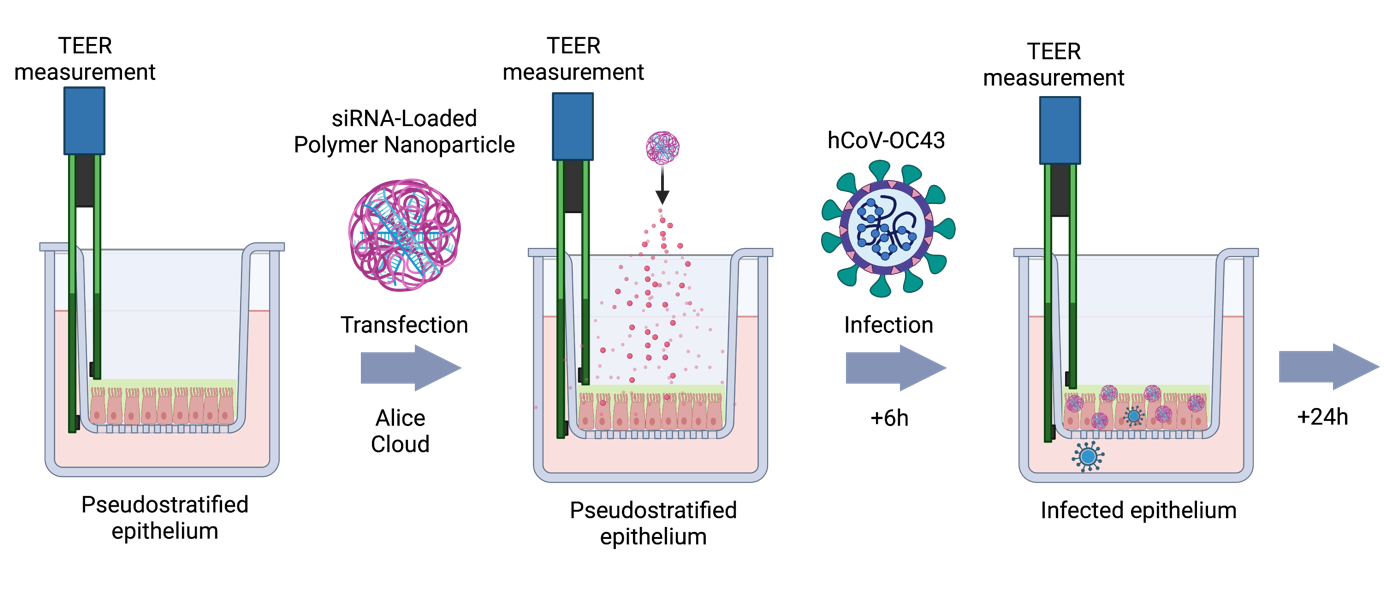
The Merkel lab is interested in smart drug delivery systems. Our research on drug and RNA delivery is subdivided into the following topics:
Synthetic nano-sized delivery systems for RNA and CRISPR/Cas
Delivery is currently the greatest hurdle for the therapeutic use of nucleic acids such as siRNA, mRNA or CRISPR plasmids and RNPs.1-4 Therefore, we develop novel delivery systems based on biodegradable and amphiphilic polymers5, 6 assisted by machine-learning approaches and prepare nanoparticles by precision microfluidic assembly7, 8 and spray drying for enhanced storage stability.3, 9, 10
Local administration routes
Due to rapid degradation by nucleases and fast excretion upon systemic injection, local administration of RNA offers more clinical relevance. By local administration, both the dose and systemic side effects can be decreased.11 We are mostly interested in pulmonary administration for local effects4, 12, 13 and nasal administration for subsequent delivery to the brain via the olfactory bulb.14
Novel safe and target-specific nanomedicines
To reach specific cell populations within the lung or to deliver nucleic acids into the brain, we attach targeting ligands to the surface of the delivery systems. These targeting moieties have a strong affinity with the target cells and tissues. Due to this high affinity, these delivery systems are also investigated for their ability to detect distant targets, such mobile immune cells, circulating tumor cells or metastases.13, 15, 16
Therapeutic approaches
Our goal is to develop novel nucleic acid-based nanomedicines for therapy of a range of diseases. We mainly focus on the treatment of respiratory virus infections,4 asthma13 and lung cancer2 with a specific focus on EGFR and Kras mutations in lung cancer and their repair for chemosensitization.
References
- Kandil, R., et al., Coming in and Finding Out: Blending Receptor-Targeted Delivery and Efficient Endosomal Escape in a Novel Bio-Responsive siRNA Delivery System for Gene Knockdown in Pulmonary T Cells. Adv Ther (Weinh) 2019, 2 (7). Awarded with the 2020 PHOENIX Pharma Science Award in Pharmaceutical Technology
- Mehta, A., et al., Targeting KRAS Mutant Lung Cancer Cells with siRNA-Loaded Bovine Serum Albumin Nanoparticles. Pharm Res 2019, 36 (9), 133.
- Zimmermann, C.M., et al., Spray drying siRNA-lipid nanoparticles for dry powder pulmonary delivery. J Control Release 2022, 351, 137-150.
- Baldassi, D., et al., Inhibition of SARS-CoV-2 replication in the lung with siRNA/VIPER polyplexes. J Control Release 2022, resubmitted unpon minor revisions requested.
- Jin, Y., et al., Synthesis and Application of Low Molecular Weight PEI-Based Copolymers for siRNA Delivery with Smart Polymer Blends. Macromol Biosci 2022, e2200409.
- Elsayed, M., et al., Influence of Oligospermines Architecture on Their Suitability for siRNA Delivery. Biomacromolecules 2014, 15 (4), 1299-1310.
- Feldmann, D.P., et al., Microfluidic Assembly of siRNA-Loaded Micelleplexes for Tumor Targeting in an Orthotopic Model of Ovarian Cancer. Methods Mol Biol 2019, 1974, 355-369.
- Feldmann, D.P., et al., The impact of microfluidic mixing of triblock micelleplexes on in vitro / in vivo gene silencing and intracellular trafficking. Nanotechnology 2017, 28 (22), 224001.
- Keil, T.W., et al., Impact of crystalline and amorphous matrices on successful spray drying of siRNA polyplexes for inhalation of nano-in-microparticles. Adv Ther (Weinh) 2021, 4 (6).
- Keil, T.W.M., et al., Characterization of spray dried powders with nucleic acid-containing PEI nanoparticles. Eur J Pharm Biopharm 2019, 143, 61-69.
- Merkel, O.M., et al., Nonviral siRNA delivery to the lung: investigation of PEG-PEI polyplexes and their in vivo performance. Mol Pharm 2009, 6 (4), 1246-60.
- Feldmann, D.P., et al., In vitro and in vivo delivery of siRNA via VIPER polymer system to lung cells. J Control Release 2018, 276, 50-58.
- Xie, Y., et al., Targeted delivery of siRNA to activated T cells via transferrin-polyethylenimine (Tf-PEI) as a potential therapy of asthma. J Control Release 2016, 229, 120-9.
- Gabold, B., et al., Transferrin-modified chitosan nanoparticles for targeted nose-to-brain delivery of proteins. Drug Deliv Transl Res 2022.
- Mohammadi, M., et al., Folate receptor targeted three-layered micelles and hydrogels for gene delivery to activated macrophages. J Control Release 2016, 244 (Pt B), 269-279.
- Jones, S.K., et al., Correlating quantitative tumor accumulation and gene knockdown using SPECT/CT and bioluminescence imaging within an orthotopic ovarian cancer model. Biomaterials 2018, 178, 183-192.

